Answer:
B. 43.40 mL
Step-by-step explanation:
We are required to determine the volume of 0.200 M Kl(aq).
We have; 155.0 mL of a 0.112 M Pb(NO3)2 solution.
We know that molarity of a solution is contained in 1 L or 1000 mL or 1000 cm³.
Hence,
- 0.112 M Pb(NO3)2 is contained in 1000 mL
- x mol Pb(NO3)2 is contained in 155.0 mL
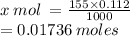
= 0.01736 moles of Pb(NO3)2
We have a mole ratio of 2: 1 (KI : Pb(NO3)2).
To obtain the number of moles of KI; we divide by half the moles of Pb(NO3)2 (aq);
(0.01736)/2
= 0.00868 moles KI
0.20 moles KI is contained in 1000 mL
0.00868 moles KI is contained in x mL
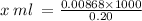
= 43.40 mL
Therefore the volume of KI required to react completely with 0.112 M Pb(NO3)2 is 43.40 mL