This is an exercise in the General Combined Gas Law.
To start solving this exercise, we obtain the following data:
Data:
- V₁ = 4.00 l
- P₁ = 365 mmHg
- T₁ = 20 °C + 273 = 293 K
- V₂ = 2,80 l
- T₂ = 30 °C + 273 = 303 K
- P₂ = ¿?
We apply the following formula:
- P₁V₁T₂=P₂V₂T₁ ⇒ General formula
Where:
- P₁=Initial pressure
- V₁=Initial volume
- T₂=end temperature
- P₂=end pressure
- T₂=end temperature
- V₁=Initial temperature
We clear for final pressure (P2)
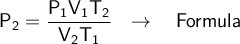
We substitute our data into the formula:
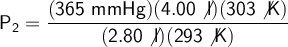
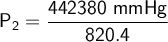
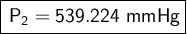
Answer: The new canister pressure is 539.224 mmHg.
{ Pisces04 }