Answer:
1. 0.35 moles NaOH
2.
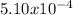 moles
moles
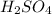
3. 505.4 g Pb
4. 1.46 g HCl (if that is wrong it's 1.5 g HCl due to sig figs)
5.
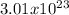 atoms of Ag
atoms of Ag
6.
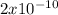 atoms of Cu
atoms of Cu
Step-by-step explanation:
1. The mass of NaOH is 40g. 22.99(Na)+16(O)+1.01(H)=40 g
14 g NaOH *
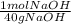 = 0.35 moles of NaOH
= 0.35 moles of NaOH
2. Mass of
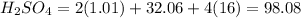
0.05 g
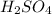 ×
×
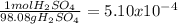
3. mass of Pb = 106.4 g
4.75 moles Pb×
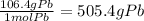
4. mass of HCl = 1.01 +35.45 = 36.36 g
0.04 mol HCl x
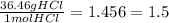
5. 5.00 mol Ag x
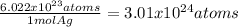
6.
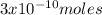 Cu x
Cu x
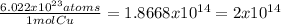 atoms of Cu
atoms of Cu