Answer:
=> 2.8554 g/mL
Step-by-step explanation:
To determine the formula to use in solving such a problem, you have to consider what you have been given.
We have;
mass (m) = 16.59 g
Volume (v) = 5.81 mL
From our question, we are to determine the density (rho) of the rock.
The formula:
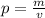
Substitute the values into the formula:

= 2.8554 g/mL
Therefore, the density (rho) of the rock is 2.8554 g/mL.