Answer:
Approximately
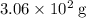 (approximately
(approximately
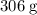 .)
.)
Step-by-step explanation:
Calculate the quantity
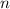 of lithium phosphate in
of lithium phosphate in
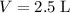 of this
of this
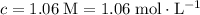 lithium phosphate solution.
lithium phosphate solution.
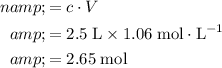 .
.
Empirical formula of lithium phosphate:
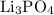 .
.
Look up the relative atomic mass of
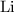 ,
,
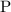 ,and
,and
 on a modern periodic table:
on a modern periodic table:
Calculate the formula mass of
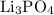 :
:
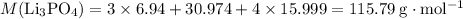 .
.
Calculate the mass of that
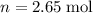 of
of
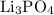 formula units:
formula units:
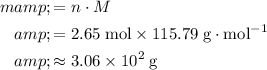 .
.