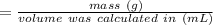Answer:
Following are the solution to these question:
Step-by-step explanation:
Please find the complete question in the attached file.
In point 1:
The information collected was given in the excel spreadsheet.
In point 2:
Each solution mass, Mass=Solution mass - empty container mass, was measured utilizing shape.
In point 3:
The density of each formula, density