Answer:
Follows are the solution to the given points:
Step-by-step explanation:
In point a:
Just the y-axis graph intensity as well as the "y" absorption. Find a path or intercept utilizing Excel or simple math.
In point b:
There are two different ways:
Graphically = Then add the unknown absorbance.
Its intensity of the unknown, if they consider the y axis to pull the straight line to the line marked but instead drop it off on the x-axis.
= resolve,
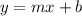 from of the line even for the x variable and also get on
from of the line even for the x variable and also get on
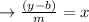
Plug
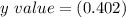 and the un-concentration is also in place
and the un-concentration is also in place