Answer:
T = 30.42°C
Step-by-step explanation:
According to the conservation of energy principle:
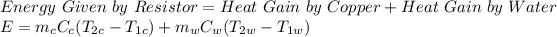
E = 120 KJ
mc = mass of copper = 13 kg
Cc = specific heat capacity of copper = 0.385 KJ/kg.°C
T2c = T2w = Final Equilibrium Temperature = T = ?
T1c = Initial Temperature of Copper = 27°C
T1w = Initial Temperature of Water = 50°C
mw = mass of water = 4 kg
Cw = specific heat capacity of water = 4.2 KJ/kg.°C
Therefore,
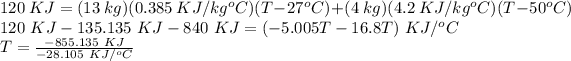
T = 30.42°C