Answer:
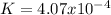
Step-by-step explanation:
Hello!
In this case, according to the given chemical reaction, it is possible to set up the equilibrium expression as shown below:
![K=([CO]^4)/([Ni(CO)_4])](https://img.qammunity.org/2022/formulas/chemistry/college/dfdzoihyweocashzu1rb5n87uyhqm4zojn.png)
Since the nickel product is solid and is not included. It means that the concentrations at equilibrium of CO and Ni(CO)₄ are:
![[CO]_(EQ)=(1.98g)/(28.01g/mol) *(1)/(2.6L)=0.0272M](https://img.qammunity.org/2022/formulas/chemistry/college/ddm34iqbcy3pwijg9senai7xaswgiiqkbf.png)
![[Ni(CO)_4]_(EQ)=(0.597g)/(170.73g/mol) *(1)/(2.6L)=0.001345M](https://img.qammunity.org/2022/formulas/chemistry/college/ptaxiflovc8ziiv0pucwd6tos5jiftkhvg.png)
Thus, there equilibrium constant for the reaction turns out to be:
Best regards!