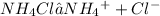Answer:
In a solution containing ammonia solution and ammonium chloride, ammonia hydrolyses in water to produce hydroxyl ions.
NH3 +H2O → NH4+ + OH-
Ammonium chloride ionises in the solution to produce ammonium ions and chloride ions where the ammonium ions undergo hydrolysis to produce more ammonia solution.