Answer: Moles of water are 0.868
Step-by-step explanation:
Mole fraction is the ratio of number of moles of one component to the total number of moles present.
moles fraction=
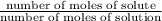
Given : mole fraction of NaCl = 0.132
Thus 0.132 moles of NaCl are present in 1 mole of solution
moles of water = moles of solution - moles of solute
moles of water = (1 - 0.132) = 0.868
Thus moles of water are 0.868.