Answer: 10. 39.7 g
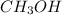
11. 650 g of
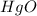
Step-by-step explanation:
To calculate the moles, we use the equation:
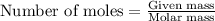
a) moles of
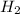
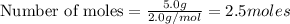
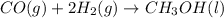
According to stoichiometry :
2 moles of
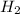 produce = 1 mole of
produce = 1 mole of
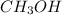
Thus 2.5 moles of
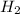 produce=
produce=
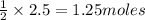 of
of
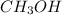
Mass of
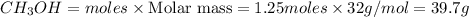
Thus 39.7 g of
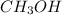 will be produced from 5.0 g of hydrogen.
will be produced from 5.0 g of hydrogen.
11.
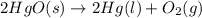
According to stoichiometry :
1 mole of
 is produced by = 2 moles of
is produced by = 2 moles of
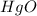
Thus 1.50 moles of
 are produced by =
are produced by =
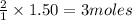 of
of
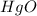
Mass of
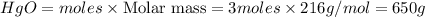
Thus 650 g of
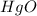 woukd be decomposed to produce 1.5 mol of oxygen
woukd be decomposed to produce 1.5 mol of oxygen