Answer:
Hydrogen is the limiting reactant.
Step-by-step explanation:
Hello!
In this case, for the identification of the limiting reactant, it is necessary to compute the yielded moles of ammonia via both hydrogen and nitrogen in agreement to the 3:2 and 1:2 mole ratios respectively:
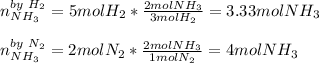
Thus, since hydrogen yields the fewest moles of ammonia we infer it is the limiting reactant.
Best regards!