Answer:
0.0474 atm
Step-by-step explanation:
Hello!
In this case, since the pressure inside the balloon is greater than that of the troposphere, the positive pressure difference is given by:
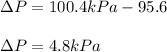
Now, since 101.325 kPa = 1 atm, the pressure in atm would be:
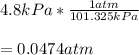
Best regards!