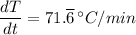Answer:
The change in temperature per minute for the sample, dT/dt is 71.
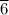 °C/min
°C/min
Explanation:
The given parameters of the question are;
The specific heat capacity for glass, dQ/dT = 0.18 (kcal/°C)
The heat transfer rate for 1 kg of glass at 20.0 °C, dQ/dt = 12.9 kcal/min
Given that both dQ/dT and dQ/dt are known, we have;
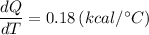
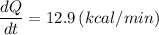
Therefore, we get;
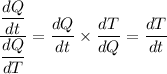
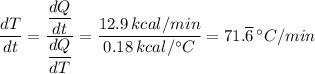
For the sample, we have the change in temperature per minute, dT/dt, presented as follows;