Answer:
39.948g Ar, or if you need to round it up then 39.95g
Step-by-step explanation:
Conversion units: 1 mole is 6.022 x 10^23 molecules, that includes atoms too.
First Step: Convert atoms to moles:
6.022 x 10^23 Ar atoms ×
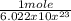 = 1 mole Ar
= 1 mole Ar
Second Step: Convert moles to grams
There is 39.948 grams in one mole of Argon so
1 mole Ar x 39.948g = 39.948g