Answer:
25 g will be left after 30,000
Step-by-step explanation:
First how many time it decays in 30,000 years: 30,000 ÷ 6000
The answer will be 5 times, it decays.
Now use the formula:
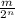 ............where m is mass and n is number of times it decays.
............where m is mass and n is number of times it decays.
Here given mass is 800 g and decays 5 times
Using the formula:
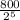
25 g