Hi there!
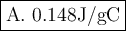
the equation q = mcΔT, where:
q = Energy (J)
cΔ = temperature change
m = mass of object
T = specific heat
Rearrange the equation to solve for the specific heat:
q/mcΔ = T
Plug in the given energy, mass, and temperature change to solve:
972 / (70 · (152 - 58)) = T
Simplify and solve:
972 / (70 · 94) = T
T = 972 / 6580 = 0.148 J/gC.