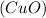Answer: The green copper (II) carbonate
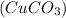 changes to black copper oxide
changes to black copper oxide
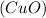
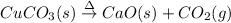
Step-by-step explanation:
Decomposition is defined as the chemical reaction in which a single compound gives two or more simple substances. It requires energy to break the bonds between reactants, thus is an endothermic process.
Thermal decomposition uses heat for decomposition.
The chemical equation for thermal decomposition of copper (II) carbonate is:
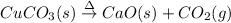
The green copper (II) carbonate
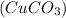 changes to black copper oxide
changes to black copper oxide