Answer:
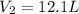
Step-by-step explanation:
Hello!
In this case, according to the given data of volume, pressure and temperature, it is possible to infer this problem can be solved via the combined gas law:
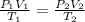
Thus, regarding the question, we evidence we need V2, but first we make sure the temperatures are in Kelvins:
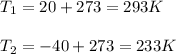
Then, we obtain:

Best regards!