Answer:
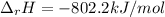
Step-by-step explanation:
Hello!
In this case, according to the given chemical reaction, it is possible to calculate the enthalpy change of the reaction via:
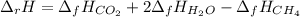
Since the enthalpy of formation of oxygen is 0. Thus, given the enthalpies of formation of gaseous carbon dioxide and water, we obtain:
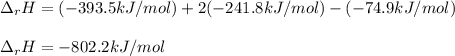
Best regards!