Answer:
584.2 g
Step-by-step explanation:
Hello there!
In this case, since we know the 20% of the atoms correspond to sodium, we can compute the total atoms as shown below:
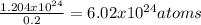
Which are also equal to 1 mol and the Avogadro's number of sodium chloride with a molar mass of 58.44 g/mol; thus, the grams of salt turn out to be:
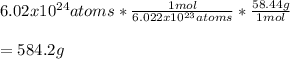
Best regards!