Answer:
4.21 moles are the moles of the gas when the volume decreases
Step-by-step explanation:
Based on Avogadro's law, the moles of a gas are proportional to its volume under pressure and temperature constants. The formula is:
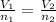
Where V₁ is initial volume (230L)
n₁ is initial moles (5.50mol)
V₂ is final pressure (176L)
And n₂ are final moles:
176L * 5.50mol / 230L = n₂ =
4.21 moles are the moles of the gas when the volume decreases