Answer : 2.24 L / 2.24 dm³
We need to write a equation for the thermal decomposition of Calcium carbonate . The decomposition is as follows ,
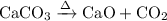
The molecular mass of Calcium carbonate is ,
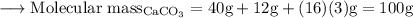
The reaction is already balanced.
- From the reaction , 100g of Calcium carbonate gives 1 mole of carbon dioxide.

- And at STP , we know that the volume of 1 mol of gas is 22.4L or 22.4 dm³ .
So that,
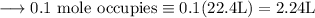
Hence ,

I hope this helps .