Answer:
b. 15.39 L
Explanation:
Given;
reacting mass of the Nitrogen gas N₂, m = 42 g
pressure of the gas, P = 2 atm
temperature of the gas, T = 250 K
molar mass of Nitrogen, N = 14 g/mol
molar mass of Nitrogen gas N₂, M = 28 g/mol
number of moles of the given Nitrogen gas;
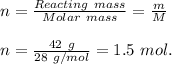
The volume occupied by the Nitrogen gas can be calculated from Ideal gas law;
PV = nRT
where;
V is the volume of the gas
R is ideal gas constant = 0.0821 atm.L / mol.K
From the equation above, make V the subject of the formula;

Therefore, the volume occupied by the Nitrogen gas is 15.39 L