Answer:
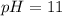
Step-by-step explanation:
Hello there!
In this case, since the ionization of the described base is:
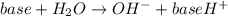
Thus, the equilibrium expression in terms of the reaction extent is:
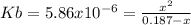
Thus, by solving for x we obtain:
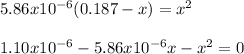
So the values of x are:
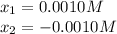
So the feasible answer is 0.0010 M, thus we compute the pOH:
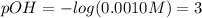
And therefore the pH:
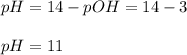
Best regards!