Answer:
Option (2)
Step-by-step explanation:
Boyle's states that at constant temperature, volume of a gas is inversely proportional to the pressure exerted by the gas.
In other words, at constant temperature, multiplication of pressure and volume of the gas is constant.
PV = k
Here 'k' = constant
From the graph attached,
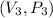 represents (0.5, 200).
represents (0.5, 200).
0.5 × 200 = k
k = 100
For pressure 'P' = 150 kPa
150 × V = 100
V =
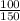
V = 0.67 L
Option (2) is the answer.