Answer:
34176.5 torr
Step-by-step explanation:
It is given that :
Initial pressure,
 torr
torr
Initial volume,
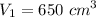
Initial temperature,
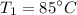
Final volume,
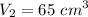
Final temperature,
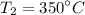
Therefore, using the combined gas laws, we can write :
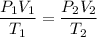
Substituting the values, we get
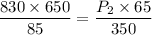
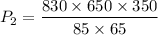
= 34176.5 torr
So the new pressure is 34176.5 torr.