Answer:
1) Fe = 69.9%
O = 31.1%
2) H = 5.19%
O = 16.5%
N = 28.9%
C = 49.5%
Step-by-step explanation:
One easy way to do percent compositions is to assume you have 100g of a substance.
1) Lets say we have 100g of Fe2O3.
The total molar mass would be:
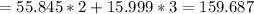
The molar mass of the Fe2 alone is:
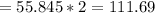
Thus, the grams of Fe2(out of a 100) could be calculated by multiplying 100g * the molar mass ratio of Fe2 to the whole:
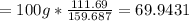
Which is approximately 69.9%.
We can find the amount of O3 by simply subtracting, as the rest of the compound is made of O3. Thus, the % composition of O3 is 31.1%
You can then do this same process to the next question, getting us the following:
H = 5.19%
O = 16.5%
N = 28.9%
C = 49.5%