Answer:
A
Step-by-step explanation:
Kw value is equal to [H30+][OH-]
In the pKw, p is -log, so we need to reverse the negative log via the following:
Kw=
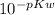
Kw=
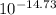
Kw= 1.9x
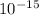
Now in pure water, [H3O+]=[OH-]
We can derive the following equation from the given, Kw=
![[H3O+] ^(2)](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/bfn6h96cbiggekd6l6vmmm78j27f1ci0zc.png) since both hydronium and hydroxide have equal concentrations.
since both hydronium and hydroxide have equal concentrations.
Substitute:
[H3O+]=
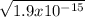
(we use square root as [H3O+] is squared)