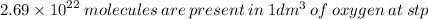Hi there!
Note: 1 mole of a any gas at ST and Pressure (273 K and 1 atm) occupies a volume of 22.4 dm^3
=> x mole of oxygen occupies 1.00 dm^3
Therefore, the mole of oxygen on 1.00 dm^3 is :
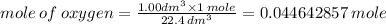
Also note that Avogadros Constant says: 1 mole of any gas contains
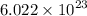 molecules.
molecules.
Hence, 0.044642857 mole of oxygen will contain :
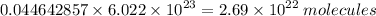
Therefore,