Answer:
The dimensions of a is L⁵·M·T⁻²mol⁻²
Step-by-step explanation:
The gas equation for a real gas, can be presented as follows;
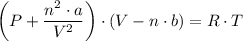
Where;
P = The pressure
V = The volume
a = A constant representing intermolecular forces
b = A constant representing molecular volume
n = The number of moles
The dimensions of the expression
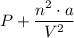 is in units of pressure, given that 'P' is in units of pressure, bar, therefore, the expression,
is in units of pressure, given that 'P' is in units of pressure, bar, therefore, the expression,
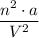 , is also measured in units of pressure
, is also measured in units of pressure
The dimensions of pressure, P = M·L⁻¹·T⁻²
'n²' unit dimension is mol², while V² is measured as liter² (L³), therefore, 'a' will convert the units of n² and V² to bars, therefore, we have;
The unit dimension of a = L²·bar/(mol²)
(L³)²·(M/(L·T²))/(mol²) = L⁵·M·T⁻²mol⁻²
The dimension of a = L⁵·M·T⁻²mol⁻²
Where;
L = Length in meters
T = Time in seconds
M = Mass in kilogram