Answer:
the unit cell edge length is 3.1585 × 10⁻⁸ cm
Step-by-step explanation:
Given the data in the question;
density = 100 / [ (84/19.3) + (16/10.22) = 100 / 5.917889 = 16.8979 g/cm³
so Aug density p_aug = 16.8979 g/cm³
Aug Atomic weight = 100 / [ (84/183.85 ) + (16/95.94) = 100 / 0.623665
= 160.3424 g/mol
now
volume = ( no. of atoms in BCC × Aug Atomic weight) / ( Aug density × Avogadro's number)
we substitute
volume = ( 2 × 160.3424 ) / ( 16.8979 × 6.023 × 10²³ )
volume = 320.6848 / 1.017760517 × 10²⁵
volume = 3.15088 × 10⁻²³ cm³
Now, unit cell edge length will be;
=
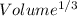
=
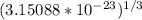
= 3.1585 × 10⁻⁸ cm
Therefore, the unit cell edge length is 3.1585 × 10⁻⁸ cm