Solution :
It is given that :
The formula of the compound =
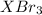
And that 4.70 g of the sample contains
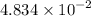 mol of Br.
mol of Br.
It means that :
1 mol of
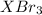 contains = 3 mol of Br
contains = 3 mol of Br
∴ 3 mol of
 contain in 1 mol of
contain in 1 mol of
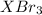
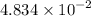 mol of
mol of
 contains
contains
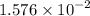 mol of
mol of
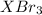
Thus the mol of
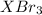 =
=
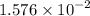 mol
mol
The given mass is = 4.700 g
Therefore, the
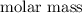 of
of
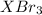
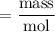
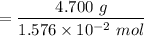
= 298.4 g/mol
So
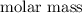 of
of
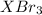 =
=
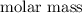 of X + 3 x
of X + 3 x
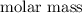 of Br
of Br
298.4 g/mol =
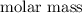 of X + 3 x 79.90 g/mol
of X + 3 x 79.90 g/mol
298.4 g/mol =
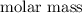 of X + 239.7 g/mol
of X + 239.7 g/mol
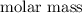 of X = 58.71 g/mol (since 1 amu = 1 g/mol)
of X = 58.71 g/mol (since 1 amu = 1 g/mol)
Therefore the atomic mass of the unknown metal = 58.71 g/mol
So the unknown meta is Nickel.