Answer: The symbol representing the result of a gas expanding against a constant pressure is W
Step-by-step explanation:
According to first law of thermodynamics, energy can neither be destroyed nor created but rather transferred to one place to another and converted to and from other energy forms.
The mathematical form of first law of thermodynamics is:
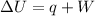
where
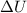 = change in internal energy
= change in internal energy
q = heat absorbed or released
W = work
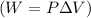
P = pressure
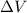 = volume change
= volume change
Thus the changes in heat or work leads to change in internal energy.