Answer:
0.012 M
Step-by-step explanation:
The reaction that takes place is:
- H₂C₂O₄ + 2NaOH → Na₂C₂O₄ + 2H₂O
First we convert 32 mg of H₂C₂O₄ to moles, using its molar mass:
- 32 mg ÷ 90 mg/mmol = 0.36 mmol
Then we calculate how many NaOH moles would react with that amount, using the stoichiometric coefficients:
- 0.36 mmol H₂C₂O₄ *
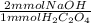 = 0.72 mmol NaOH
= 0.72 mmol NaOH
Finally we divide NaOH moles by the used volume, to calculate the molarity:
- 0.72 mmol NaOH / 60.9 mL = 0.012 M