Answer:

Step-by-step explanation:
We are asked to find the molar mass of C₃H₈O₂.
The molar mass is the mass of 1 mole of a substance. The values are found on the Periodic Table because they are equivalent to the atomic masses. However, the units are grams per mole instead of atomic mass units.
Look up the molar masses of the individual elements in the compound: carbon, hydrogen, and oxygen.
- Carbon (C): 12.011 g/mol
- Hydrogen (H): 1.008 g/mol
- Oxygen (O): 15.999 g/mol
Look at the subscripts in the formula. There is a subscript of 3 after C, another of 8 after H, and another of 2 after O. Therefore, there are 3 moles of carbon, 8 moles of hydrogen, and 2 moles of oxygen in every mole of the compound. Multiply the element's molar mass by the subscript.
- C₃: 12.011 * 3 = 36.033 g/mol
- H₈: 1.008 * 8 = 8.064 g/mol
- O₂: 15.999 *2=31.998 g/mol
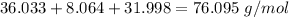
Round to the 3 significant figures. For the number we calculated, that is the tenths place. The 9 in the hundredth place to the right tells us to round the 0 up to a 1.
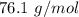
The molar mass of C₃H₈O₂ is approximately 76.1 grams per mole.