Answer:
The empirical formula is the simplest form;
Given:
Oxygen O at 94.1% and
H at 5.9%
Assume 100grams.
94% = 0.941 x 100gm. = 94.1 gm x 1mole/16gm. = 5.88 moles of O
5.9% = 0.059 x 100gm. = 5.9gm. X 1moleH/1.002gm. = 5.88 moles of H
There is one mole of O for each mole of H so the empirical formula is
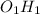
and written as OH.