Answer:
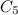
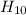
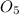
Step-by-step explanation:
Each CH2O unit has a molar mass of (12 + 2*1 + 16) = 30 g/mole.
The actual compound has a molar mass of 150 g/mole. That means it has 5 CH2O units, since 150/30 = 5
The molecular formula must be
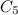
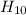
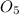
C: 5*12 = 60
H: 10*1 = 10
O: 2*16 = 80
Total = 150 g/mole