Solution :
The buffer is the one that contains weak acid (the
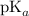 is nearly equal to the required pH) and the salt of its conjugate base.
is nearly equal to the required pH) and the salt of its conjugate base.
The
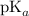 values of the given weak acids are as follows :
values of the given weak acids are as follows :
HCOOH :
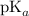 = 3.744
= 3.744
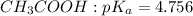
HCN :
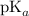 = 9.21
= 9.21
Since the required pH is 5.03, the suitable buffer is the mixture of the acetic acid and the salt of its conjugate base.
Let suppose the volumes of
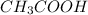 and
and
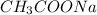 are x and y mL respectively.
are x and y mL respectively.
So the total volume of the buffer is 1000 mL.
∴ x+ y = 1000 ................(1)
Writing the Henderson-Hasselbalch equation for the given buffer solution :
![$pH = pK_a + \log \ ([CH_3COONa])/([CH_3COOH])$](https://img.qammunity.org/2022/formulas/chemistry/college/nxo1g4dbpzm4u6z31rqae02tpy7f5ar3q0.png) .............(2)
.............(2)
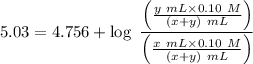
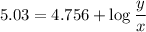
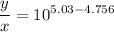
y = 1.9 x
Substituting the values of y in equation (1), we get
x + 1.9 x = 1000
x = 345
Putting the value of x in (1), we get
345 + y = 1000
y = 655
Therefore the volume of
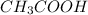 is 345 mL and the volume of
is 345 mL and the volume of
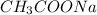 is 655 mL.
is 655 mL.