Answer:
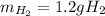
Step-by-step explanation:
Hello!
In this case, since the metal is not given, we can set up the following chemical reactions:
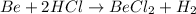
Thus, since the HCl is a 2:1 mole ratio with hydrogen, we can use the following setup to calculate the required mass of hydrogen:
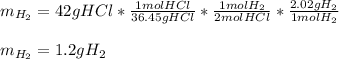
Best regards!