Answer:
Rate = 116m⁻¹s⁻¹[lactose][H]⁺
Step-by-step explanation:
the formula for rate of reaction is given as
Rate = k[lactose]∧α[H]⁺∧β
we solve for the value of α and β
([lactose]₁/[lactose]₂)∧α
α =
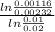
when we divide this equation
α =
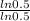
α = 1
we find β
R₁/R₂ = 0.01/0.02(0.001/0.001)∧β
0.00116/0.00232 = 0.5(1)∧β
β = 1
Rate = k[lactose]∧α[H]⁺∧β
we have to find the value for k
k = 0.00116/0.01(0.001)
k = 0.00116/0.00001
= 116m⁻¹s⁻¹
Rate = 116m⁻¹s⁻¹[lactose][H]⁺