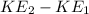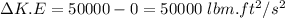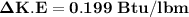Answer:
Step-by-step explanation:
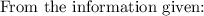
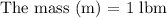
Suppose: g = 32.2 ft/s²
At the inlet conditions:
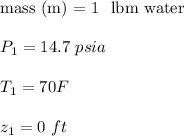
At the outlet conditions:
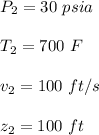
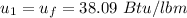
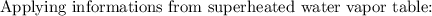
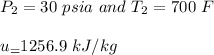
The change in the internal energy is:
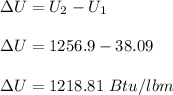
For potential energy (P.E):
Initial P.E = mgz
P.E = 1 × 32.2 × 0 = 0 ft²/s²
Final P.E = mgz
P.E = 1 × 32.2 × 100 = 3220 ft²/s²
The change in the potential energy = PE₂ - PE₁
ΔPE = (3220 - 0) ft²/s²
ΔPE = 3220 ft²/s²
ΔPE = (3220 × 3.9941 × 10⁻⁵) Btu/lbm
ΔPE =0.12861 Btu/lbm
Initial Kinetic energy (K.E)
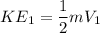
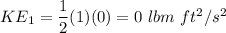
FInal K.E
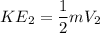
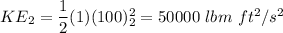
Change in K.E
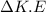 =
=