Answer:
a) 570 kWh of electricity will be saved
b) the amount of SO₂ not be emitted or heat of electricity saved is 0.00162 ton/CLF
c) $1.296 can be earned by selling the SO₂ saved by a single CFL
Explanation:
Given the data in the question;
a) How many kilowatt-hours of electricity would be saved?
first, we determine the total power consumption by the incandescent lamp
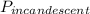 = 75 w × 10,000-hr = 750000 wh = 750 kWh
= 75 w × 10,000-hr = 750000 wh = 750 kWh
next, we also find the total power consumption by the fluorescent lamp
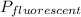 = 18 × 10000 = 180000 = 180 kWh
= 18 × 10000 = 180000 = 180 kWh
So the value of power saved will be;
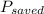 =
=
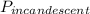 -
-
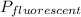
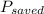 = 750 - 180
= 750 - 180
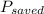 = 570 kWh
= 570 kWh
Therefore, 570 kWh of electricity will be saved.
now lets find the heat of electricity saved in Bituminous
heat saved = energy saved per CLF / efficiency of plant
given that; the utility has 36% efficiency
we substitute
heat saved = 570 kWh/CLF / 36%
we know that; 1 kilowatt (kWh) = 3,412 btu per hour (btu/h)
so
heat saved = 570 kWh/CLF / 0.36 × (3412 Btu / kW-hr (
heat saved = 5.4 × 10⁶ Btu/CLF
i.e eat of electricity saved per CLF is 5.4 × 10⁶
b) How many 2,000-lb tons of SO₂ would not be emitted
2000 lb/tons = 5.4 × 10⁶ Btu/CLF
0.6 lb SO₂ / million Btu = x
so
x = [( 5.4 × 10⁶ Btu/CLF ) × ( 0.6 lb SO₂ / million Btu )] / 2000 lb/tons
x = [( 5.4 × 10⁶ Btu/CLF ) × ( 0.6 lb SO₂ )] / [ ( 10⁶) × ( 2000 lb/ton) ]
x = 3.24 × 10⁶ / 2 × 10⁹
x = 0.00162 ton/CLF
Therefore, the amount of SO₂ not be emitted or heat of electricity saved is 0.00162 ton/CLF
c) If the utility can sell its rights to emit SO2 at $800 per ton, how much money could the utility earn by selling the SO2 saved by a single CFL?
Amount = ( SO₂ saved per CLF ) × ( rate per CFL )
we substitute
Amount = 0.00162 ton/CLF × $800
= $1.296
Therefore; $1.296 can be earned by selling the SO₂ saved by a single CFL.