Answer:
262.44 grams of HCl is contained in 2.40 L of a 3.0 M solution.
Step-by-step explanation:
Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a certain volume.
Molarity is calculated as the number of moles divided by the volume of the solution:
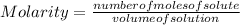
Molarity is expressed in units
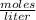 .
.
In this case:
- Molarity= 3 M
- Number of moles of solute= ?
- Volume of solution= 2.4 L
Replacing:
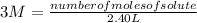
and solving you get:
number of moles of solute= 3 M* 2.40 L
number of moles of solute= 7.2 moles
Being the molar mass of HCl 36.45 g/mole, then you can apply the following rule of three: if 1 mole has 36.45 grams, 7.2 moles how much mass does it have?
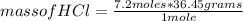
mass of HCl= 262.44 grams
262.44 grams of HCl is contained in 2.40 L of a 3.0 M solution.