Answer:
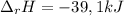
Step-by-step explanation:
Hello!
In this case, since it is possible to assume that the heat released by the reaction is absorbed by the water in the calorimeter we can write:
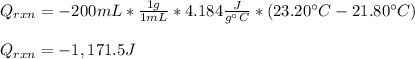
Now, since the reaction between silver nitrate and hydrochloric acid is:
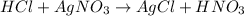
We can see there is a reacting 1:1 mole ratio, thus, the reacting moles are computed via the molarity of the solutions:
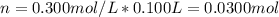
Finally, the enthalpy of reaction is:
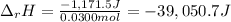
And in kJ:
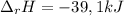
Best regards!