Answer: due to bond pair - lone pair and lone pair -lone pair repulsion
Step-by-step explanation:
In
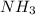 , nitrogen is the central atom and there are 3 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be
, nitrogen is the central atom and there are 3 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be
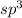 and geometry of the molecule will be pyramidal as there are three bond pairs and one lone pair.The bond angle for pyramidal geometry is 107°. The bond angle reduces due to lone pair bond pair repulsions.
and geometry of the molecule will be pyramidal as there are three bond pairs and one lone pair.The bond angle for pyramidal geometry is 107°. The bond angle reduces due to lone pair bond pair repulsions.
In
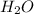 , oxygen is the central atom and there are 2 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be
, oxygen is the central atom and there are 2 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be
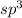 and geometry of the molecule will be bent as there are two bond pairs and two lone pairs.The bond angle for pyramidal geometry is 104.5°.The bond angle reduces further due to greater lone pair-lone pair repulsions.
and geometry of the molecule will be bent as there are two bond pairs and two lone pairs.The bond angle for pyramidal geometry is 104.5°.The bond angle reduces further due to greater lone pair-lone pair repulsions.