Answer:

Step-by-step explanation:
The relationship between moles and gases is unique. Regardless of the type of gas, there will always be 22.4 liters of gas in 1 mole, as long as it's at STP (standard temperature and pressure).
We can make a ratio using this information.
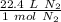
Multiply by the given number of moles.
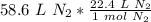
Flip the fraction so the liters of nitrogen cancel.
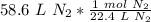
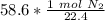
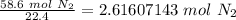
The original measurement of liters has 3 significant figures, so our answer must have the same.
For the number of moles calculated, 3 sig figs is the hundredth place. The 6 in the thousandth place tells us to round the 1 to a 2.
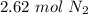
58.6 liters of N₂ gas at STP is equal to about 2.62 moles.