Answer:
The final temperature of the gas would need to be approximately 158.4 K
Step-by-step explanation:
The details of the sample of nitrogen gas are;
The initial temperature of the nitrogen gas, T₁ = 22.7°C = 295.85 K
The initial volume occupied by the gas, V₁ = 12.2 L
The initial pressure of the gas, P₁ = 150.4 kPa
The final volume of the gas, V₂ = 9.7 L
The final pressure of the gas, P₂ = 101.3 kPa
Let 'T₂', represent the final temperature of the gas, by the ideal gas equation, we have;
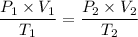
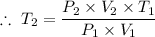
Plugging in the values gives;
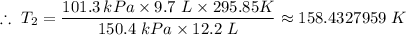
The final temperature of the gas, T₂ ≈ 158.4 K