Answer:

Step-by-step explanation:
To convert from mass to moles, the molar mass must be used. This can be found on the Periodic Table.
- Gold (Au): 196.96657 g/mol
Use this number as a ratio.
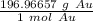
Multiply by the given number of grams.
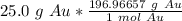
Flip the fraction so the grams of gold will cancel.
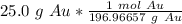
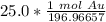
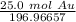
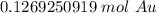
The original measurement has 3 significant figures, so the answer must have the same. For the number we calculated that is the thousandth place. The 9 in the ten thousandth place tells us to round the 6 to a 7.
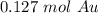
25.0 grams of gold is about 0.127 moles of gold.